Anticorrosive agents
An anticorrosive agent temporarily or constantly protects materials from corrosive attack. In the case of surfaces which are in constant contact with a corrosive medium, especially water (e.g. in a cooling or heating circuit), the use of anticorrosive agents is often inevitable.
Antifreeze protection
The term antifreeze protection comprises all measures and methods which are to prevent the freezing of liquids such as water. This is necessary because the volume of ice becomes larger than the volume of the liquid water and thus the required space, which is increased by a few per cent, can create large forces (frost bursting).
Antifreezing agents
An antifreezing agent is a product (e.g. on the basis of glycol) which lowers the freezing point of another compound (e.g. water).
Boiling point
The boiling point is the temperature at which a liquid compound or product becomes gaseous.
Cooling brine
The cooling brine is a liquid whose freezing point is below the freezing point of water. Cooling brines are used for the cold transport from a central refrigerating plant to different refrigeration consumers. It is typically used for air conditioners, cold chains or technical refrigerating plants. In the past, saline solutions were often used as cooling brines. This is also where the German name of the brine (“Sole”) comes from. Due to the distinctive corrosive properties of saline solutions, especially with regard to the use in metal systems, modern products today are offered on the basis of glycol, for example.
Ethylene glycol
(Mono)Ethylene glycol (MEG) (trivial name glycol) is the simplest bivalent alcohol with the chemical name ethane- 1,2-diol. It is the simplest diol.
Freezing point
The freezing point is the temperature between the physical conditions “solid” and “liquid”. The best known freezing point is the freezing point of water. On the temperature scale in degree Celsius, it defines the zero point. If a “freezing point” is mentioned without any further information, it normally refers to pure water, meaning 0 °C. The freezing point defines the conditions of the transformation of a material from the liquid to the solid state, which is called “freezing”. If it is important that a liquid also stays liquid when the temperature is below its freezing point, there are special frost protection agents available which can lower the freezing point, e.g. of water, down to -55 degree Celsius.
Frost protection agents
A frost protection agent is a product (e.g. on the basis of glycol) which lowers the freezing point of another compound (e.g. water). The effect depends on the used product and its concentration. It is often used as additive to cooling systems or solar liquids. Frost protection agents can be bought as concentrates or as premixed solutions. Apart from e.g. glycol, alcohol or glycerine, corrosion inhibitors and stabilisers (which are already included in brand products) are absolutely necessary.
Glycol
Glycols are part of the group of alcohols (alkanols) and are also called bivalent alcohols due to two hydroxyl groups. The simplest bivalent alcohol is ethane diol (ethylene glycol) with the elemental formula C 2 H 6 O 2. Glycols can often be found in frost protection agents, coolers and de-icers because their melting points are -10 to -15° and therefore lower than the melting point of water. In connection with water, the melting point is significantly lower and can reach down to -55° if the mixture is correct. Glycols are used as solvents or disinfectants e.g. in varnishes and adhesives. Other glycols are e.g. diethylene glycol, triethylene glycol, propylene glycol, dipropylene glycol etc.
Glycol/water mixture
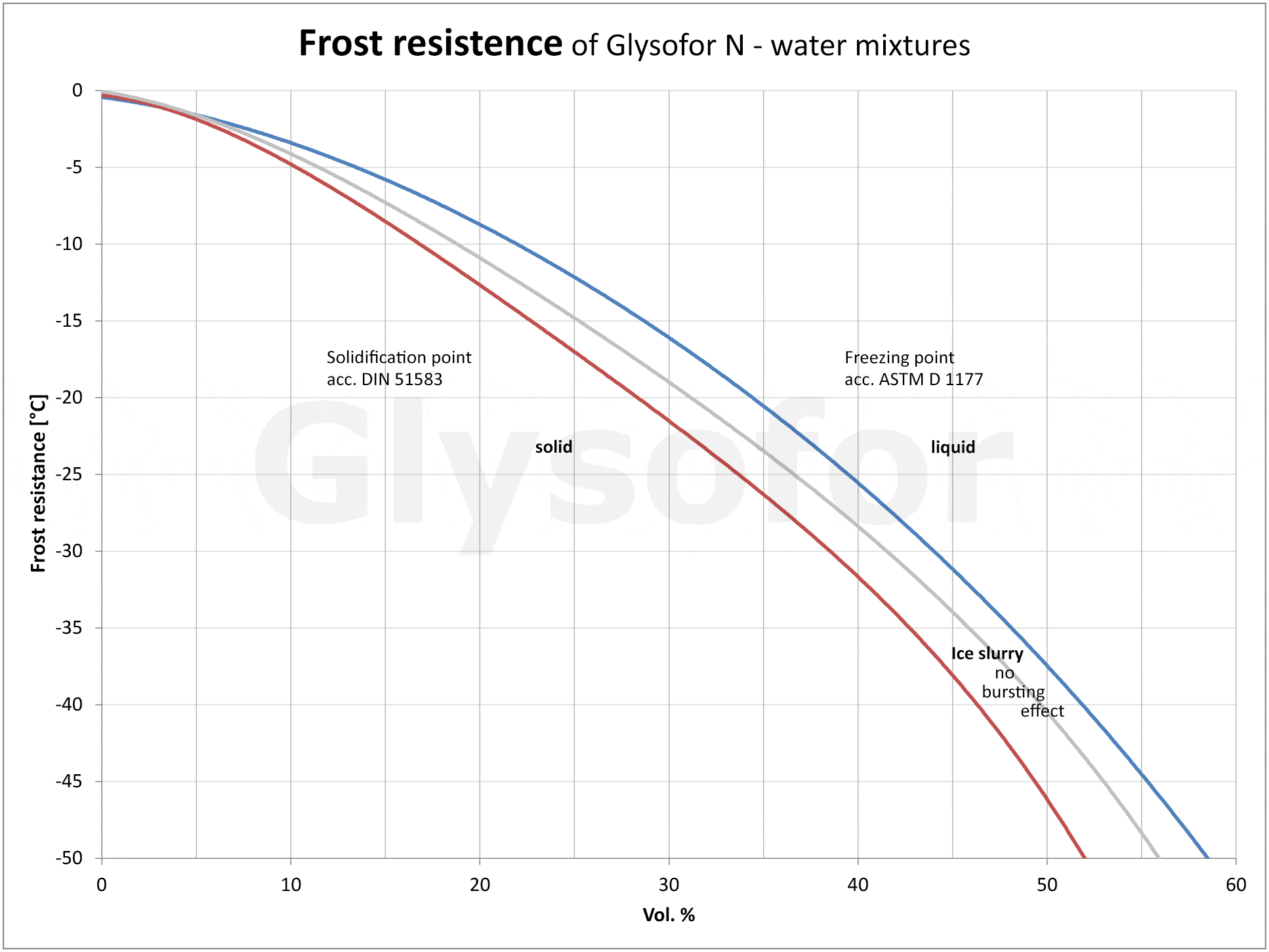
The function of the glycols in a glycol/water mixture is to lower the freezing point. However, not only the freezing point is reduced; glycols have an additional effect when used in water, as illustrated in Figure [1] using the example of the monoethylene glycol-based product Glysofor N. The mixture does not freeze at the specified freezing point, rather it initially forms ice slush. At the crystallization point, small, spherical ice particles are produced, which also remain continuously in motion, so that ice slush is not able to cause any metallic components of the system to burst. If the temperature drops below the solidifying point, bursts are possible.
The glycols used are primarily ethylene glycol and propylene glycol. Ethylene glycol exhibits the best physical properties when used as antifreeze in heat transfer media. When using glycol in cooling systems in the food processing industry or if the cooling medium could come into contact with drinking water, on the other hand, only propylene glycol (1,2-Propanediol), which is approved in the EU as a food additive, may be used.
The secret is in the mix
Refrigeration or heating systems should not be filled with pure glycols or antifreeze concentrates. For example, the freezing point of pure ethylene glycol is only -13 °C. In the ideal mixing ratio with water, on the other hand, a reduction of the freezing point to under -50 °C can be achieved.
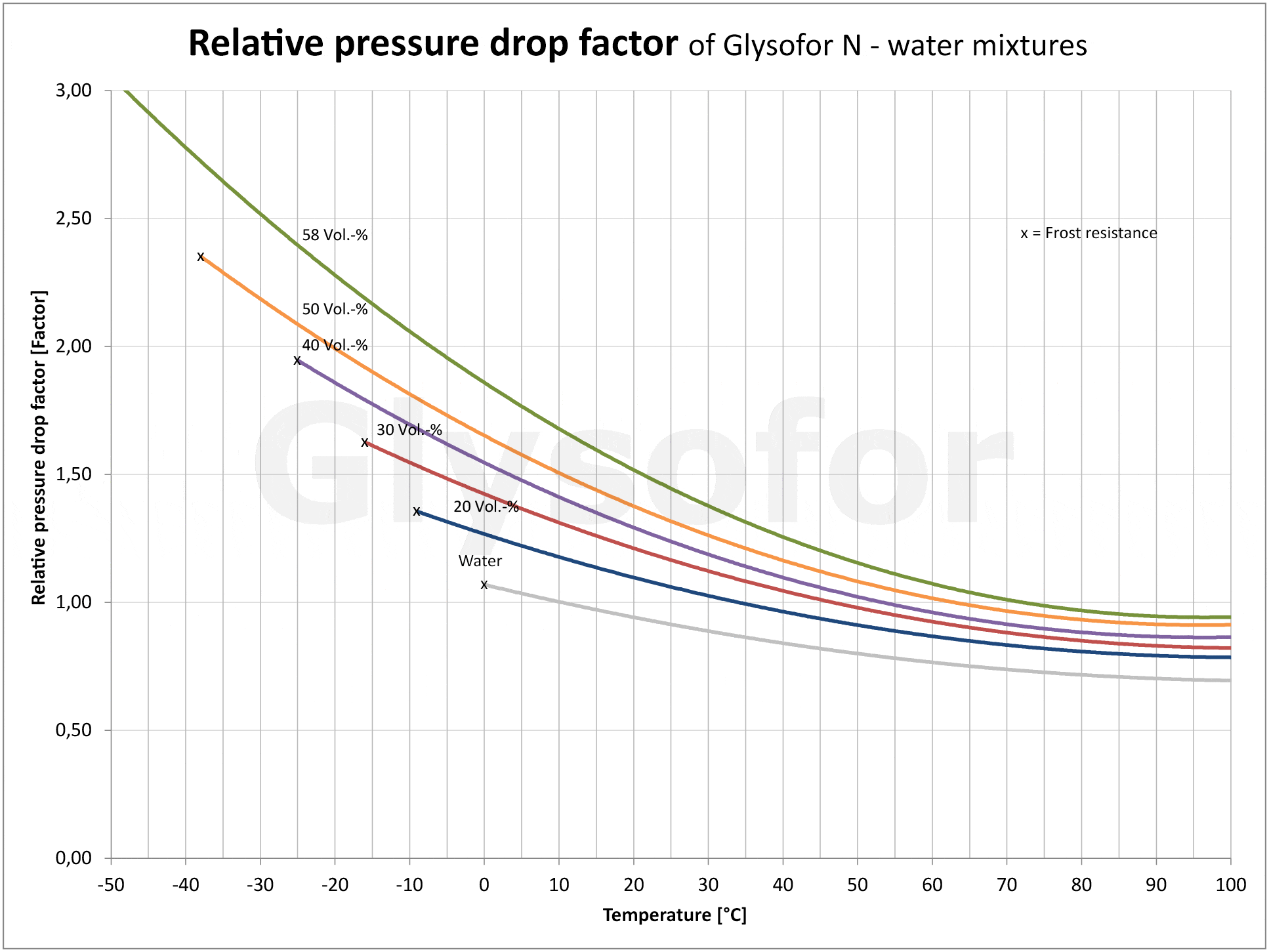
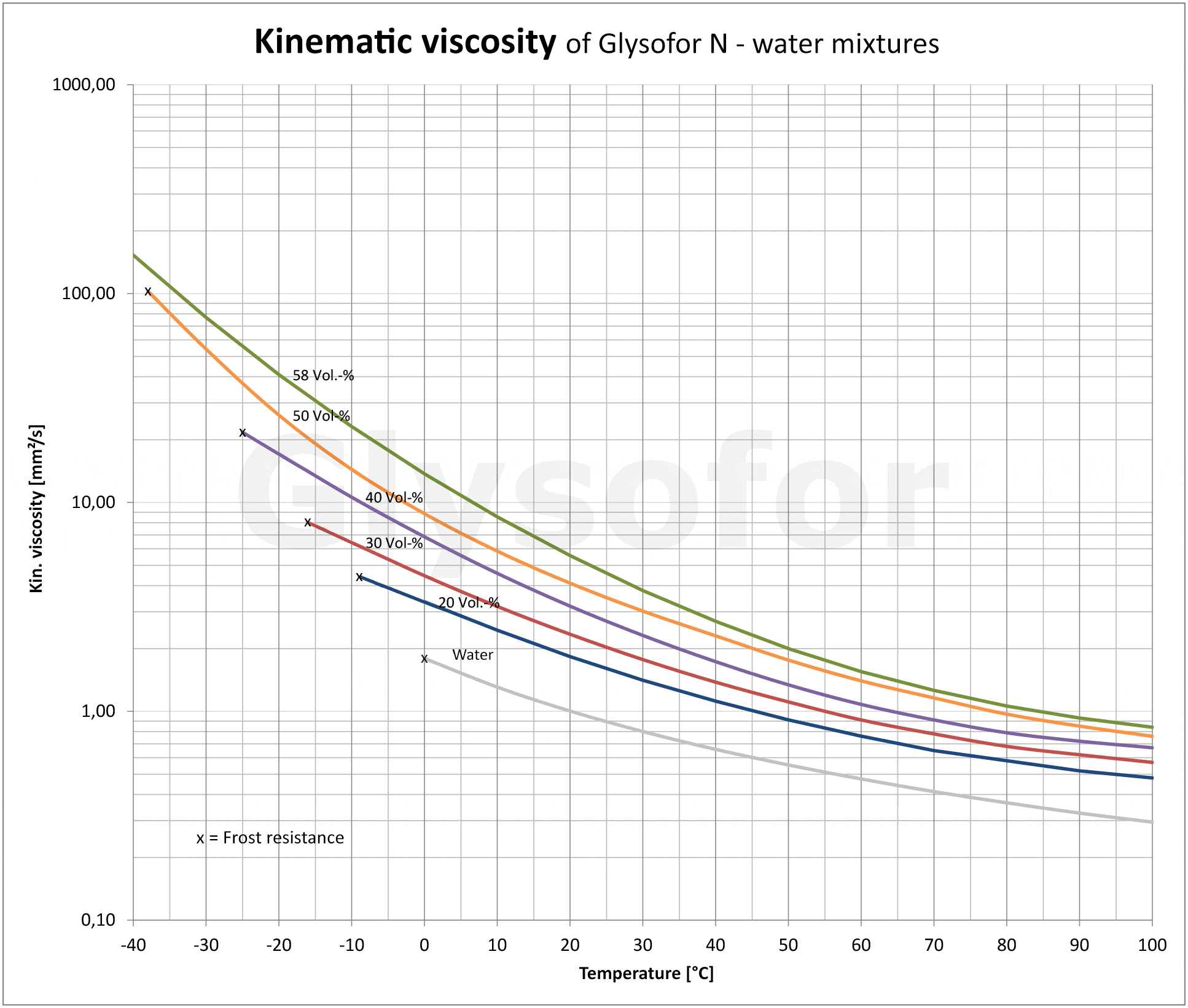
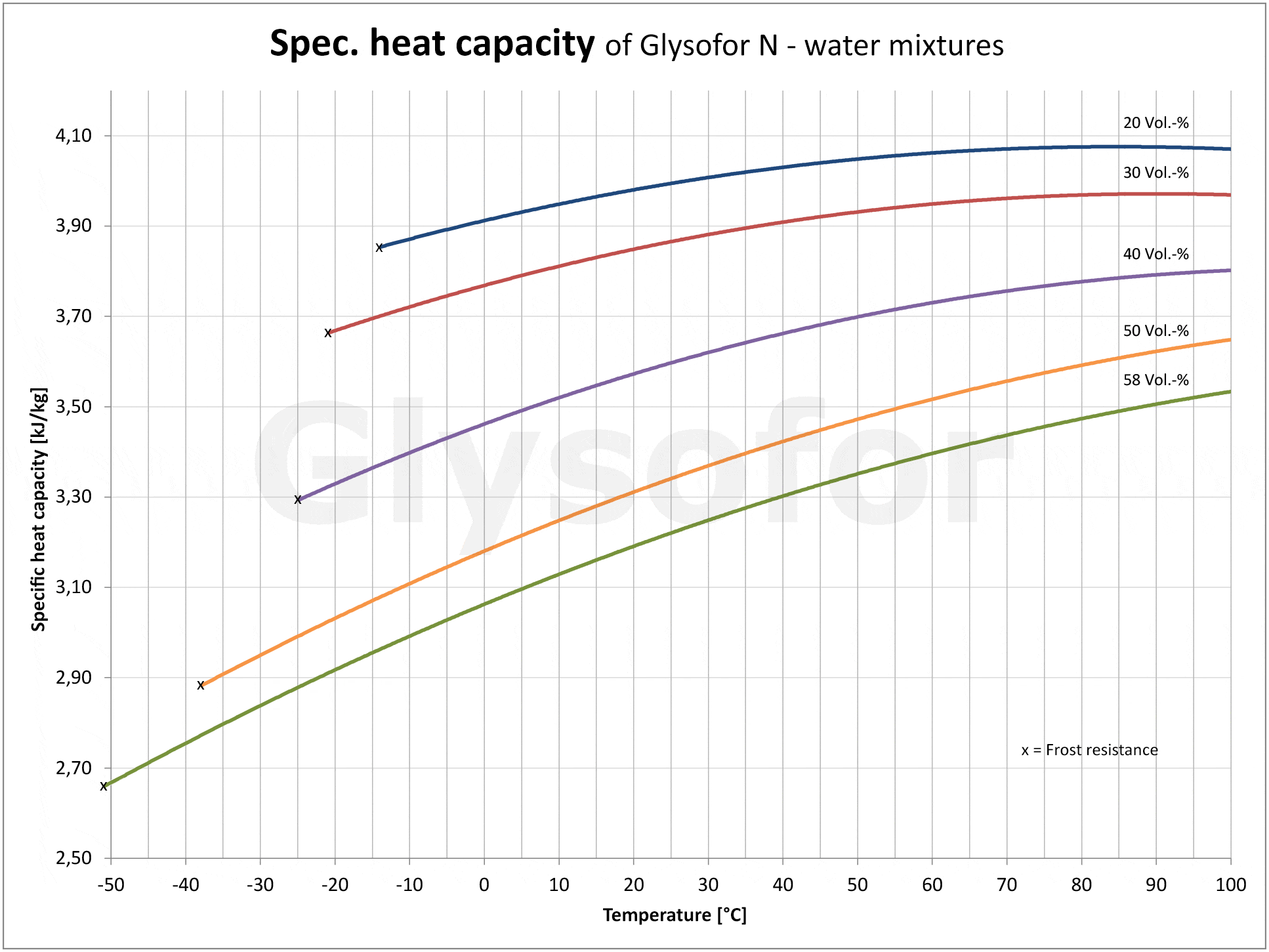
As you can see in the following illustrations using the example of the monoethylene glycol-based product Glysofor N, as the proportion of glycol increases so does the relative pressure drop [2] and the kinematic viscosity [3]. A pump must compensate for these drops with a greater delivery height. The higher density of the glycols compared to pure water has an effect on the drive performance of the motor, and consequently on energy consumption. The lower specific heat capacity [4] (thermal absorption capacity) of the glycol results in reduced performance from the system.
For this reason, usage conditions should be checked in advance in order to determine the level of frost resistance required.
Follow this rule: As much as necessary and as little as possible!
Heat carriers
Heat carriers or heat transfer media are media which transport heat from a place with a higher temperature to a place with a lower temperature (in a cooling or heating circuit). Depending on the purpose and the temperature range, heat carriers are also called heating fuels (heating media), refrigerant agents or cooling agents. Heat carriers are usually called refrigerant agents if they are used for applications below 0 °C.
Heat conductivity
The heat conductivity (thermal conductibility) of a liquid is its ability to transport thermal energy in the form of heat. The (specific) heat conductivity in W/(K•m) is a temperature-dependent constant.
Ice slurry
Ice slurry is a mixture of small ice particles (0.01 to 0.5mm), water and a material or product which lowers the freezing point. Ice slurry is liquid to semiliquid and pumpable. It is produced when the temperatures of a liquid are close to its specific freezing or solidification point and, due to its condition, does not have any explosive effect as long as it is able to flow freely.
Multi-metal installation
A multi-metal installation can be a heating or cooling system made of different metals. Typical metals of a multi-metal installation are copper, brass, aluminium, steel, iron, grey iron, soft solder and hard solder.
Propylene glycol
1,2-propane diol (1,2-propylene glycol) is a clear, transparent, nearly odourless and very hygroscopic liquid. Propylene glycol belongs to the multivalent alkanols. It can be mixed with water in every ratio, but not with fatty oils. Propylene glycol is industrially produced by the hydrolysis of propylene oxide. In this connection, a high temperature method without catalysis at 200-220° is used. Propylene glycol is used as a wetting agent and plasticiser in sanitary products such as skin creams, toothpaste and antiperspirants. Propylene glycol can significantly improve the solubility of different compounds and guarantee a more stable dispersion of drugs in ointments. Furthermore, it can often contribute to a significant resorption improvement of different active ingredients. Propylene glycol is used in the food industry as a carrier and carrier solvent of colourants, antioxidants, emulsifiers and enzymes. Due to its harmlessness, it is contained in heat transfer and refrigerant media in the food processing industry (Glysofor L), frost protection agents, cooling brines, solar liquids etc. Propylene glycol is used as additive in the food of dairy cattle and authorised in the EU as food additive (E 1520).
Refractometer
A refractometer for frost protection agents is a measuring device which determines the content of a frost protection agent in a solution. With the help of a refractometer, frost protection solutions can be regulated and tested in a quick and precise way.
Solar liquid
The solar liquid transports – in the case of plants filled with liquid – the heat of the generator to the consumer or accumulator. It consists of water and a special product which lowers the freezing point of the water and raises the boiling point. This means that the plant is protected from frost damage if the temperature is lower and the transformation of the solar liquid to a gaseous state is delayed if the temperatures of the collector are high. If the temperatures are high and the solar liquid in the collectors becomes gaseous, the plant comes to a standstill and the developing vapour pressure is generally absorbed via safety vessels (expansion vessels). The condition and – if required – the exchange of the solar liquid is to be checked during the maintenance procedure. Modern products such as Glysofor Solar are non-toxic and environmentally friendly. The higher the concentration, the lower the temperatures the plant can tolerate without damage. A concentration with an active content of more than 50% should be avoided as it lowers the specific heat capacity of the mixture. The pump will not be sufficiently cooled either. The viscosity of the mixture and thus the required pump work and current consumption are increased. All in all, this leads to a lower efficiency of the plant. In extreme cases, the pump can have difficulties starting up. If the plant is exposed to very low temperatures, an ice slurry is generated if the glycol content is sufficient. However, this ice slurry does not destroy the pipes.
Solidification point
The solidification point is the temperature at which a liquid loses its flowability and becomes solid.
Triethylene glycol
Triethylene glycol is produced by the ethoxylation of diethylene glycol and is a side product in the production of ethylene glycol. Due to its high boiling point, it is used for applications which require high temperatures (> 200 °C). It is an important interstage product in the production of polyester resins. Due to its odour-neutralising properties, triglycol is used in air sanitizers. Triglycol also dries the ambient air and has dehydrating effects on micro-organisms. With the help of triglycol, the ambient air in hygiene areas is sanitised. Triethylene glycol is also used in brake and hydraulic systems in order to raise the boiling point of a liquid. Furthermore, it is used to dehumidify offshore natural gas.
Viscosity
The viscosity of a fluid is a measure of its resistance to gradual deformation. The higher the viscosity, the more viscous (less capable of flowing) the liquid and the lower the viscosity, the less viscous (more capable of flowing) the liquid.